


Next: Induced Emission
Up: Einstein's Coefficients
Previous: Spontaneous Emission
Contents
Absorption
Photon with energy 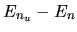 is absorbed by the atom in the
state of n.
At the same time the atom is excited from the state n to n
is absorbed by the atom in the
state of n.
At the same time the atom is excited from the state n to n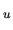 .
The number of this transition is proportional to the number of
atoms in the state n,
.
The number of this transition is proportional to the number of
atoms in the state n, 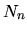 , the number of photons passing and
a cross-section of this absorption.
This is written as
, the number of photons passing and
a cross-section of this absorption.
This is written as
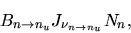 |
(2.135) |
where 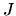 represents the average specific intensity of radiation with
corresponding wavelength.
This coefficient is called Einstein's
represents the average specific intensity of radiation with
corresponding wavelength.
This coefficient is called Einstein's 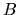 coefficient for absorption.
coefficient for absorption.
Kohji Tomisaka
2007-11-02