


Next: Spontaneous Emission
Up: Radiative Transfer
Previous: Radiative Transfer Equation
Contents
Einstein's Coefficients
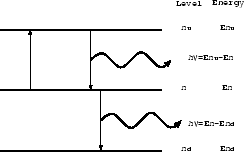
Figure 2.11:
 |
In this section, we describe
the absorption coefficient 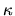 and the emissivity
and the emissivity 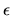 for the line emission and absorption.
Here, consider a hypothetical atom which has only three levels
named n
for the line emission and absorption.
Here, consider a hypothetical atom which has only three levels
named n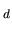 , n, n
, n, n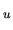 .
and assume the energy of each level is, respectively,
.
and assume the energy of each level is, respectively, 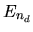 ,
, 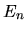 ,
and
,
and 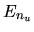 .
When the atom changes its state from n
.
When the atom changes its state from n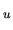 to n,
an photon with energy of
to n,
an photon with energy of
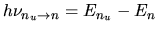 is emitted,
if this transition is radiative.
Besides the radiative processe, atom changes its state exchanging
its energy with other atoms, molecules and electrons.
is emitted,
if this transition is radiative.
Besides the radiative processe, atom changes its state exchanging
its energy with other atoms, molecules and electrons.
Subsections
Kohji Tomisaka
2007-07-08
